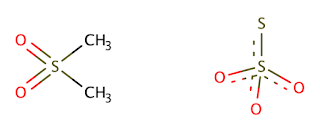In the past I've already spoken about how much of the Jan Van Eyck Academy's 'Future Materials Bank' programme is somewhat misguided.
In that post I didn't point fingers at individual contributors, because I do believe the general aim of the Future Materials Bank is a positive one and I don't like to deride people who make honest mistakes.
That being said, I recently visited their website again and one of their featured recent additions was listed as 'Phenol, Sodium Ascorbate'.
This caught my eye, as phenol is extremely toxic and definitely not safe for use outside a laboratory. Naturally it occurred to me that this is in stark contrast with the aims of the Future Materials Bank.
The accompanying article is confusingly written and contains a number of errors, so I was surprised to learn at the end of it that its author, Giulia Principe, was a 'tech fellow' at the Rijksakademie in 2022. The tech fellow programme was instituted that same year and gives a small number of 'artists with advanced knowledge of materials and/or techniques' the possibility 'to become a technical specialist. [...] In addition, the tech fellow conducts research into materials or technology in the workshop(s), and makes a report'. The year-long fellowships are supported with a budget of €22.000 for each of the fellows.
The article at the Future Materials Bank seems to be derived from Principe's research results of this fellowship as published on the website of the Rijksakademie. That text is somewhat easier to read, yet contains the same errors, so I will base my criticism here on that short paper.
Principe's 'research' was concerned with finding more environmentally friendly solutions to photographic film development. The main body of the text is thus split in four parts: Developer, Stop, Fixer and Waste Management.
The first sentence of the developer section already is a good indication of things to come: 'All film developers are made from phenols, a molecule that consists of a phenyl group bonded to
a hydroxyl group.' None of this is strictly speaking true. Most commercial film developers are in fact made from phenols, but this is because phenols are good reducing agents for silver halides. In theory any reducing agent can work as a film developer, so even something like iron(II) can be, and has been, used as such. The second part of the sentence confuses the nomenclature, as phenols are benzene rings with one or more hydroxyl substitutes. When a benzene ring is itself a substituent on another molecule, then it's called phenyl-. This confusion of nomenclature also helps explains the strange indication of 'phenol' as a 'future material', as phenols, multiple, are a class of molecules that can have many different properties. Some of those molecules are helpful and essential to complex life, while others, like simple phenol, are dangerous and toxic.
The rest of the section is full of these kind of statements that miss the mark and they do nothing but show Principe's own lack of understanding of the things she is professing.
The main 'result' of her 'research' is that you can use ascorbic acid as a film developer. This has been known, as Principe herself asserts, since at least the 1930's, so this is hardly news. A clear understanding of what makes a good reducing agent might have lead her to some other possibilities of compounds that could act as successful film developers, but no explanation is given by her as to why ascorbic acid works well as a film developer. Neither is there an outline of what kind of properties a good film developer should have. The only theoretical background she presents is a screenshot of another paper, that shows an image of two isomers of ascorbic acid. She doesn't provide any form of citation or context for this paper. The screenshot does include some clarifying text by its authors, however this is cut-off mid sentence when it gets to the clarifying part.
Directly after Principe merely repeats that 'the addition of sodium carbonate is necessary to render the ascorbic acid solution alkaline in
order for it to work properly' and no explanation is given why this is would be so. No proportions are given by which these two should be mixed, not a single bit of elucidation is presented in these 'research results' as to why or even how one could use ascorbic acid to develop film. Nor is there so much as a broad outline of what is happening when film is exposed and developed.
For the record, developing film means that an electron is added to (a cluster of) silver ions, which then become permanent and visible silver metals. All film developers are thus molecules that are able to donate electrons which selectively reduce the exposed Ag+ to Ag0. Film developers are thus all reducing agents, with some other characteristics, like being soluble in water. Ascorbic acid is a particularly good reducing agent because of the resonance with the double bond next to the hydroxyl group, which partially stabilises the negative charge in the ascorbate ion. Phenolic compounds are even better reducing agents for this purpose because the conjugated pi-orbitals in the aromatic ring have even greater resonance stabilisation. This reaction needs to happen in basic conditions because the ascorbic acid will remain neutrally charged unless it's deprotonated. All of this is still a somewhat clunky explanation that requires more elaboration to most readers, but at the very least it gives some indication of what is happening and why.
In her project Principe also attempted to combine the ascorbic acid with extracts from plants, as in her words 'any source from the fungi or plant kingdom will add something to the developer'. Again no theoretical explanation or reasoning is given and the whole section is pretty difficult to follow. It can however be gleaned that she is somehow searching for phenolic compounds from 'natural' sources. 'After testing several phenolic sources, I found a successful combination of sodium ascorbate and phenols extracted from blueberries. It works perfectly and just as fast as a regular developer with both film and paper.' This extraction from the blueberries apparently happened through simple macerating and boiling. I found it bold, if not brazen, to claim that it's specifically the phenolic contents of blueberries that are extracted and contributing to this reaction. As we have already seen, any reducing agent is a possible film developer. Most acids are reducing agents, some sugars as well, and berries are full of those. Proper extraction and characterisation of wanted analytes is its own field of study, so to claim that you isolated a certain mildly water-soluble group of compounds by nothing but some soaking is questionable at best. Principe's own adventure into this complex field is when she realised that 'the extraction process can involve ethanol depending on the polarity of the source'. I'm not exactly sure what 'the polarity of the source' is supposed to mean in this specific context, but her next assertion that 'certain natural phenols have different polarities and they can be extracted by solvent with opposite polarity', is unfortunately false. All phenols are somewhat polar due to the high electronegativity of the oxygen atom, so the use of 'certain natural phenols' here is a clear safeguard against strong claims which might be wrong. As for the second part of the sentence, solvents tend to dissolve molecules of similar polarity, not the opposite. A clear example is the inability to mix water and oil, as water is a very polar substance, and oil an a-polar substance.
The developer section of the text ends abruptly, with no real conclusion other than the previously cited unbased assertion that 'it works perfectly and just as fast'.
Even the most elementary lab reports contains some theoretical background on the performed experiment, together with a detailed, and preferably reworkable, description of the experimental method(s) used, the raw results of those experiments and a discussion of those results. All of this is referenced with a bibliography of cited sources. This quote-unquote report contains none of these.
But we are only halfway through the text, so maybe it will get better.
The next section, stop, is very short. There is some talk about how even though it isn't considered harmful it should still be replaced, as 'waste may have to be pre-treated before discharge and should not be discharged to the septic system.' The manufacturer likely included this message because they know their product will be used to wash away other chemicals, but they don't know which chemicals, in what manner, or in what quantity. Very likely harmful candidates are bromide salts originating from the silver bromide in the film and perhaps the aforementioned harmful phenols. Thus replacing the stop doesn't necessarily change anything about its environmental effects.
Nevertheless, according to Principe, 'replacing the stop was easy, since the solution should be simply acidic in order to work.' Finally we have found something that is unequivocally true in this text, but unfortunately she offers no explanation as to why it is true. As we have already seen before, the stop introduces a (weak) acid to the development solution in order to (re)protonate the ascorbate, at which point it is no longer active as a reducing agent. It can then simply be washed away.
The next chapter, fixer, is then regrettably one big pile of misinformation.
The main assertion of the section is that 'a low-toxicity photographic fixer can be made from just salt and water'. This is just not true and we will discuss why in a second. First it must be addressed that the most common fixer is ammonium thiosulphate, which, as Principe says 'is not classified as environmentally hazardous. However,' she continues, 'it is classified as a Hazardous Decomposition Product: heating to dryness will cause production of ammonia, oxides of sulfur, ammonium sulfate and sulfur.' The key phrase there is 'heating to dryness'. If your aqueous solution of ammonium thiosulphate is boiled until all water has been evaporated and only some hot powder is left, then, and only then, will such decomposition occur. As commercial photographic fixer is used in water at room temperature, such a decomposition event will never never happen. There thus is no danger in using ammonium thiosulphate.
Yet according to Principe it is still necessary to replace this fixer and her preferred replacement is salt, or sodium chloride. Sodium chloride is itself an ionic bond of Na+ and Cl-. The reaction that thus supposedly occurs with silver bromide should be AgBr (s) + NaCl (aq) → AgCl (aq) + NaBr (aq). A very clear indication that this cannot and will not happen is that AgCl, or silver chloride, is itself insoluble in water and that NaCl is in fact often used to precipitate Ag[NO3] out of solution.
But perhaps we're wrong in this straightforward assertion of knowledge that can be found in the solubility charts taught in high school chemistry.
In Principe's description, 'at least 40% of the solution should be salt' and it will take '24 to 48 hours to clear the film'. The extremely high concentration of NaCl and the long reaction time should also be indications that using table salt might not be a solution to the problem.
Principe claims that 'the water should be saturated with salt'. As the definition of saturation is the maximum amount of a substance that can be dissolved in another, it should be obvious that saturating a solution with one substance is always counterproductive if the goal is to get another substance into that solution.
For a more mathematical example as to how ridiculous this proposition is, let's remember that the proposed reaction has a stoichiometric ratio of 1:1, where one molecule of AgBr reacts with one molecule of NaCl. AgBr weighs about 3,2 times as much per mole than NaCl does, so with one gram of NaCl, you can theoretically dissolve 3,2 grams of AgBr. In a picture of the process, some film strips are sitting in about 250 mL of water. If 40% of the solution, by weight I assume, is NaCl, then that 250 mL of water contains 100 grams of salt. That 100 grams of salt, if Principe's assertions were somehow true, could then theoretically react with about 320 grams of silver bromide. For some reason I doubt that a little five centimetre strip of photographic film contains 320 grams of silver bromide.
But, you might say, it has already been said that the reaction is slow, so that's why you need so much salt. This ignores the fact that the reaction can only happen at the surface of the film strip, so all those other molecules that aren't present at that surface don't contribute to the reaction. Hence the need for agitation in any of the steps in film development.
In an attempt to speed up the reaction, Principe also considers the temperature. She quotes the Arrhenius equation, which broadly says that for a 10 ºK increase in temperature, the reaction rate roughly doubles. This has mostly to do with activation energy, or the amount of energy needed to start a reaction, which is not necessarily relevant here. In her text she somehow gets to a very specific increase of the rate of 2,7 times at 30 ºC. She thus obviously attempted to calculate something, although its not clear to me what exactly that could be. As we have already seen that in the salt solution there is barely any reaction happening at all, a far simpler explanation is that solubility tends to increase with higher
temperatures. You can see this when you add sugar to warm tea and it readily dissolves, but then when the tea cools down, most of this sugar will become a sticky lump at the bottom.
Clearly there are problems with the feasibility of Principe's proposed reaction, that's why 'thanks to the Rijksakademie resources, I was able to work with a chemist over the summer to find a sustainable way to increase the velocity of the fixer'. I'm not sure what the background of this supposed chemist was, or what instructions they were given, but despite them not convincing Principe in the errors of her ways, they nevertheless gave some possible solutions that may have some impact in getting the AgBr to dissolve.
The simplest of these was the addition of methylsulfonylmethane (MSM). Once again Principe gives no explanation as to why this would work, but she does go on about how it has 'many claims of uses in healthcare and beauty products'. It also supposedly 'speeds up the reaction to 5 hours'. Although not a great result per se, we can still see why it might do something if we compare the structures of methylsulfonylmethane and thiosulphate:
As you can see, there are some similarities in their composition, with a number of oxygen atoms bonding to a central sulphur atom. Even if in MSM the oxygen atoms aren't negatively charged, they still have two free electron pairs that may draw in some of the positively charged silver ions. The effectiveness of MSM without experimental measurement is to be debated however, as even sulphite, or SO32-, which can be considered an intermediate between sulphonyl and thiosulphate, only poorly dissolves Ag+ according to our trusty solubility chart. However, since the MSM is probably present in a large excess and the amount of silver to be diluted is miniscule, it might actually be something that works, albeit not very effectively.
A final suggestion that Principe makes for the fixer recipe is adding potassium bromide, that according to her will speed up the process and 'also dissolve the silver bromide'. The only mechanism by which potassium bromide can react directly with silver bromide is: AgBr + KBr → AgBr + KBr. Clearly this is a pointless tautology, but Principe nevertheless asserts that 'adding 25 grams of potassium bromide to the salt fixer speeds up the process.' If this is experimentally found to be true, then it might do this by reacting with some other molecule that reacts more strongly with bromide and would otherwise hinder the reaction with silver. However, in more general cases, the presence of an excess of bromide will impede any other reaction with the silver in what is known as the common ion effect.
So now we have clearly seen that it can't be NaCl, or KBr, that dissolves the silver bromide, then what might be the mechanism at work here? A suggestion I've found which sounds plausible is that the silver bromide reacts with the small amounts of anti-caking agents present in industrially produced table salt. As these additives are generally larger ligands that usually are able to dissolve metals to some extent, there might be a possibility that the small amounts present in the saturated table salt solution are enough to dissolve some of the silver bromide over the course of a day. However, I'm not fully convinced of this explanation, as the only legal anti-caking agents in the EU are likewise poorly soluble in water when combined with silver. It could be equally as likely that the slow dissolution process Principe observes is simply the time it takes for the miniscule amount of silver to dissolve in pure water. It is unclear, however, whether or not this possibility has been tested or even considered.
In the final chapter, waste management, it once again becomes abundantly clear that Principe understands very little of the chemistry she's working with. She firstly claims that 'because of the organic nature of these chemical alternatives, waste management is easier to
handle, which is a non-sequitur, especially if one considers the use of the word organic, as meaning carbon-based, in chemistry. Shortly after she asserts that 'discarding fixer solutions is more difficult due to the traces of silver left in the spent solution'. Her first answer to this problem is electroplating the trace amounts of silver out of solution. This is technically possible, if not very effective, but she completely overlooked a very big problem with this method when applied to her fixer recipe. Principe's fixer recipe is a saturated solution of sodium chloride. Electrolysis of sodium chloride will mostly produce chlorine gas, which was famously used as a chemical weapon during the first world war. So although the tiny amounts of silver might have some environmental impact, electrolysis is definitely not the answer to that particular problem.
In her report Principe never considers the side products of her reactions, which are usually also the waste products. After all, these are the things that aren't part of your desired reaction, but are nevertheless present. Bromide, for example, is never mentioned by her as a waste product, even if halogens are commonly a major consideration in processing laboratory waste.
To come back to the problem of silver waste; an obvious answer is making the silver highly soluble, with, say, thiosulphate. In a 2009 review of the bioaccumulation and toxicity of silver compounds, H.T. Ratte already concluded that 'silver thiosulfate, a highly soluble compound and main component of
wastewaters of photoprocessors, has a very low toxicity (e.g., it is
15,000–17,000 times less toxic than silver nitrate). This can be
attributed to the silver complexed by thiosulfate, which reduces the
bioavailability of free silver ions.'1 So if diluted and refreshed properly, using the commercial fixer is most likely the easiest, best, safest and most environmentally friendly solution. There's really very little reason to be complicating matters much further.
In conclusion, Guilia Principe's 'research' merely posits a single well-known fact from the 1930's. In addition to a number of false or incomplete assertions, the only novel solution she presents cannot work in the way she claims it does and the first method she suggests for handling the waste products produces a deadly gas.
She spent a year and twenty-two thousand euros to come to these conclusions, which were supported by a major institution in the form of the Rijksakademie and were mindlessly regurgitated by another in the form of the Jan van Eyck Academie.
I would like to end on positive note about all this, but considering their lofty claims of expertise I unfortunately can't see it as anything other than an embarrassment for everybody involved.
References
1.
Ratte, H.T. (1999), Bioaccumulation and toxicity of silver compounds: A
review. Environmental Toxicology and Chemistry, 18: 89-108. https://doi.org/10.1002/etc.5620180112