The starting point for this post is the article 'Ze zeggen dat de wereld kleurlozer is geworden' by Dutch artist Barbara Collé. The article is a passionate, but meandering, treatise on the increasing presence of dull and grey colours in industrial production.
While I agree with many of the observations that she makes in regards to subjective colour experience, the arguments she employs to make her points tend to be confused and disjointed.
In particular I would like to focus on one section of the article, which elaborates on the observation of Hella Jongerius that colour in design has gotten more flat over the decades. This fading of colour is attributed to 'carbopigment', established in the next sentence as Carbon Black. It isn't clear whether this attribution is made by Jongerius or Collé, but what follows are a number of wrongly construed statements that make an emotional appeal against the use of Carbon Black.
Collé states that Carbon Black is an 'industrial product' that consists of 'soot particles of heavy petroleum and coal tar'. It seems like this phrasing is based on a poor translation from English to Dutch for various descriptions of related carbon-based compounds, because as written it's mostly incorrect. While Carbon Black is the result of incomplete combustion of hydrocarbons, it's distinguished from soot through size and chemical composition. Furthermore, both heavy petroleum products and coal tar are characterised by their high content of aromatic rings, while Carbon Black is mostly aliphatic. The harmful connotations we have with petroleum and tar are caused in a large part by their high aromatic content, which is absent in Carbon Black. Your average campfire is going to produce very similar particles and to describe Carbon Black in this way is thus misleading at best.
Collé's principal complaint against Carbon Black is that it 'kills' and 'blunts' colours when used in mixtures with other pigments. She claims that it becomes clear why this dulling effect exists if you consider that Carbon Black is also used to make 'plastic' UV-resistant. 'Because characteristic of this soot pigment is that it completely doesn't react to light', she says. 'It kills every colour. The industry loves this and calls dead 'colour-fast' and 'reliable'.'
The arguments she makes here are simply incongruent. Carbon Black is used to make polypropylene more uv-resistant, but this property has no inherent relation to colour. To see that uv-resistance isn't related to colour, simply consider your average sun cream. Sunscreens obviously have a high resistance to ultraviolet light, but most of them are transparent to somewhat white in colour, not the dulling dark grey we would expect from Collé's argument.
Collé's arguments seem to depend on a misguided understanding of what light is, how it reacts with various substances and how this affects our perception of the colour of these substances.
What we call light is more formally called electromagnetic radiation. The energy of which is related to its wavelength through the equation E = hv/λ, where E is energy, h is Planck's constant, v is the speed of light and λ is the wavelength. Because both h and v are constants that are divided by the wavelength, we can say that the shorter a wavelength is, the higher the energy will be. Through historically grown customs, we give different names to electromagnetic radiation of different wavelengths, but other than their wavelengths, x-rays do not fundamentally differ from visible light, microwaves or radio waves.
Visible light has a wavelength ranging from ~380 nanometres, where we see it as violet, and ~700 nanometres, where we call it red. The wavelengths of the ultraviolet spectrum are shorter than those of visible light, ranging from ~200 to ~380nm. Because they are shorter they are higher in energy and therefore more damaging.
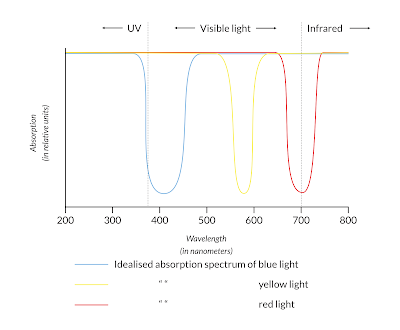
The absorption of electromagnetic radiation by a compound in the spectrum of ultraviolet to infrared is important in the chemical analysis of compounds. For reasons we won't go into here, single atoms absorb light at singular wavelengths, and molecules absorb in broader 'bands' of wavelengths, related to their atomic composition. In UV-Vis absorption spectrometry, the relative absorption of UV and visible light is measured and plotted out in a graph. In such a graph, the relative absorbance of electromagnetic radiation on the y-axis is plotted against the wavelength on the x-axis in the following manner:
These observations are in clear contradiction with both claims of Collé that Carbon Black 'kills' colour because it is UV-resistant and that it doesn't react with light.
Both Carbon Black and indigo carmine readily absorb UV light. Yet as you may or may not know, indigo fades relatively quickly with exposure to UV radiation and is therefore the opposite of UV-resistant. Resistance to ultraviolet light is thus not related to how much UV radiation a compound absorbs, but instead how it will release that energy afterwards. Indigo is more prone to chemical decomposition through its central double bond, while Carbon Black is a larger, more stable molecule that is more likely to dissipate the absorbed energy through a process called vibrational relaxation.
Furthermore, from Carbon Black's absorption throughout the spectrum we can infer that it does in fact react to visible light of all wavelengths. To put it more succinctly, this reactivity is precisely what makes it black. Carbon Black's reactivity to light, as Collé calls it, thus doesn't affect the appearance of mixtures with Carbon Black in the way she supposes.
She further claims that 'carbon' 'kills every colour' when it's added to paints 'to make other pigments darker'.
To make a pigment darker is to increase the absorption of the range where the pigments normally emits, or doesn't absorb, light. Say we have a pigment that absorbs all light from exactly 200 to 575 nm and from 585 to 800 nm. This would make it very yellow. If we were to darken this pigment, ideally we would add a substance that only absorbs light between 575 and 585 nm, a kind of anti-yellow. Such a substance does not exist, for any colour. No two pigments will have exactly complementary absorptions and even if they did, mixing them perfectly will be essentially impossible. Thus you can't uniformly darken a colour, but the best alternative we have is adding black pigments, of any sort, which absorb throughout the visible spectrum. They increase the absorption of all wavelengths, thereby relatively increasing the absorption of previously unabsorbed wavelengths more. This is thus the closest to what one could call a strict darkening of a colour.
Collé instead describes an alternative process, which 'every classical painter knows', where pigments with other colours, like red and green, are added to yellow to achieve an olive green colour. Which is fine, but in that case you haven't darkened your original yellow, you simply selectively increased the absorption of red and blue light. This will naturally give a more complex absorption spectrum, but it will also be one that has unrecognisably transformed your original yellow. You might get a mixture that can 'play a game with light' in such a case, but it won't be a darkening of yellow. In her argument she is thus comparing apples to oranges.
Collé's text ultimately is a rationalisation of her opinion that duller colours are less beautiful than brighter, more 'complex' ones. On the whole I would agree with that sentiment, but I can't abide when people use scientificly sounding rethoric to make a point that at its core is emotional and psychological.